In the long-term co-evolution and interplay between insects and microorganisms, diverse interaction relationships and regulatory mechanisms have developed, including mutualistic symbiosis, pathogenic parasitism, and immune defense. Mosquitoes serve as vectors for various diseases such as malaria and dengue fever. Research on the interactions and mechanisms between mosquitoes and microorganisms provides a theoretical foundation and technical support for developing novel strategies for mosquito-borne disease control.
Wang Lab focuses on mosquitoes and their key interacting microorganisms, using biological interactions as the central theme. By integrating multidisciplinary approaches such as molecular biology, epigenetics, cell biology, immunology, chemical ecology, and microbiomics, we aim to explore the following:
- Mechanisms of mosquito immune defense and pathogen infection and transmission.
- Molecular mechanisms of gut microbiota homeostasis, mutualistic symbiosis, and their mediation of host-pathogen interactions in insects.
- Epigenetic regulatory mechanisms underlying mosquito-microbe interactions.
- Mechanisms of mosquito reproductive behavior and chemical communication.
- Development of new mosquito-borne disease control strategies based on interaction relationships.
Mechanisms of mosquito reproductive behavior and chemical communication
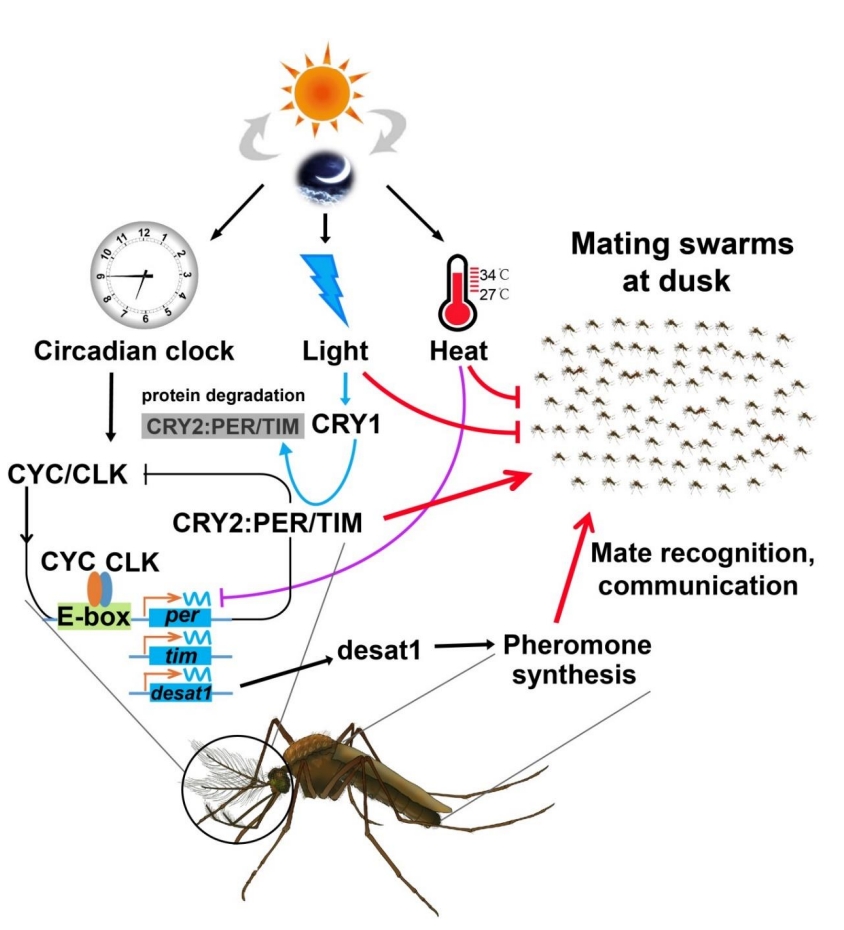
We deciphered the molecular machnism of swarm mating and courtship communication in malaria mosquitoes
We show that mosquito swarming and mating are coordinately guided by clock genes, light, and temperature. Transcriptome analysis shows up-regulation of the clock genes period (per) and timeless (tim) in the head of field-caught swarming Anopheles coluzzii males. Knockdown of per and tim expression affects Anopheles gambiae s.s. and Anopheles stephensi male mating in the laboratory, and it reduces male An. coluzzii swarming and mating under semifield conditions. Light and temperature affect mosquito mating, possibly by modulating per and/or tim expression. Moreover, the desaturase gene desat1 is up-regulated and rhythmically expressed in the heads of swarming males and regulates the production of cuticular hydrocarbons, including heptacosane, which stimulates mating activity.
Wang, G., et al. (2021). Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science, 371(6527), 411-415. DOI: 10.1126/science.abd4359
Gut bacteria and their mediation of host-pathogen interactions in insects
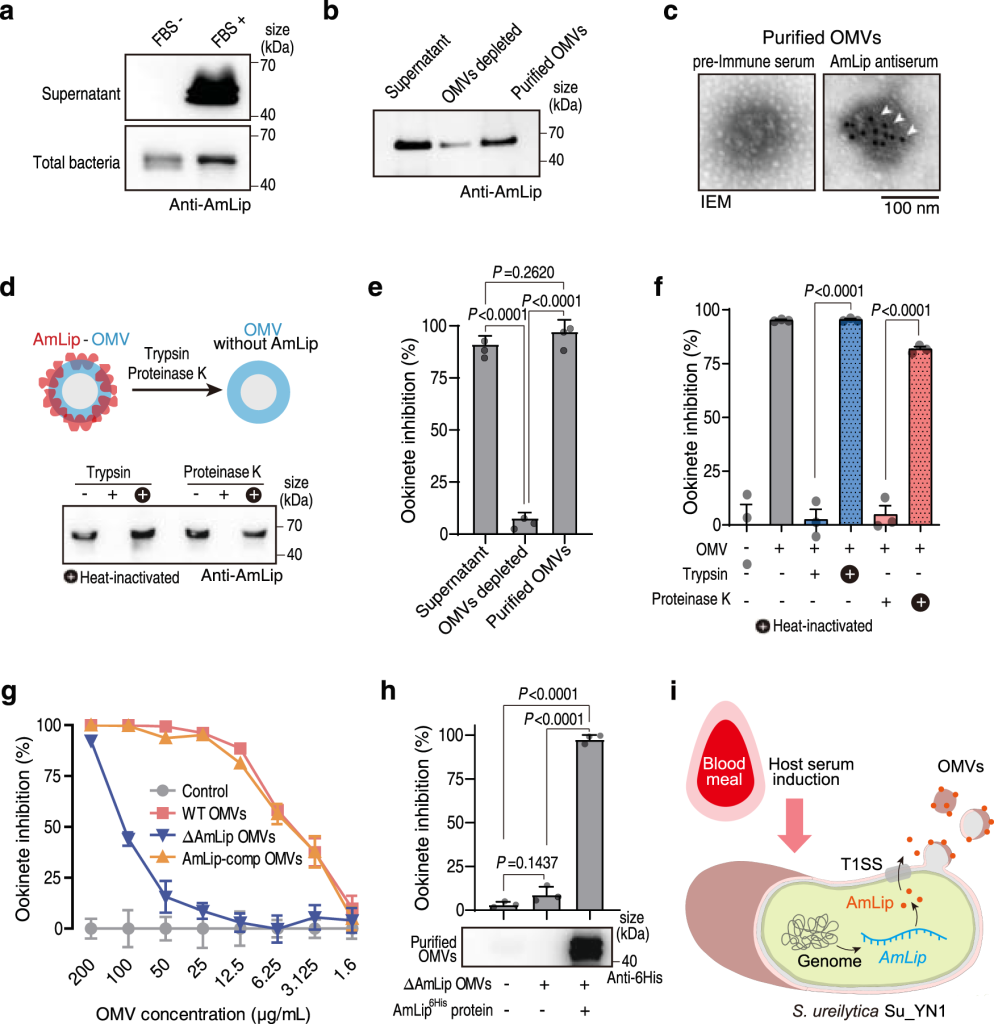
We found a natural Plasmodium blocking symbiotic bacterium Serratia ureilytica Su_YN1 that delivers the effector lipase AmLip to Plasmodium parasites via outer membrane vesicles (OMVs). After a blood meal, host serum strongly induces Su_YN1 to release OMVs and the antimalarial effector protein AmLip into the mosquito gut. AmLip is first secreted into the extracellular space via the T1SS and then preferentially loaded on the OMVs that selectively target the malaria parasite, leading to targeted killing of the parasites. These findings reveal that this gut symbiotic bacterium evolved to deliver secreted effector molecules in the form of extracellular vesicles to selectively attack parasites and render mosquitoes refractory to Plasmodium infection. The discovery of the role of gut commensal-derived OMVs as carriers in cross-kingdom communication between mosquito microbiota and Plasmodium parasites offers a potential innovative strategy for blocking malaria transmission.
Gao, H., et al. (2023). Outer membrane vesicles from a mosquito commensal mediate targeted killing of Plasmodium parasites via the phosphatidylcholine scavenging pathway. Nature Communications, 14(1), 5157.
Fungal pathogenesis in insects
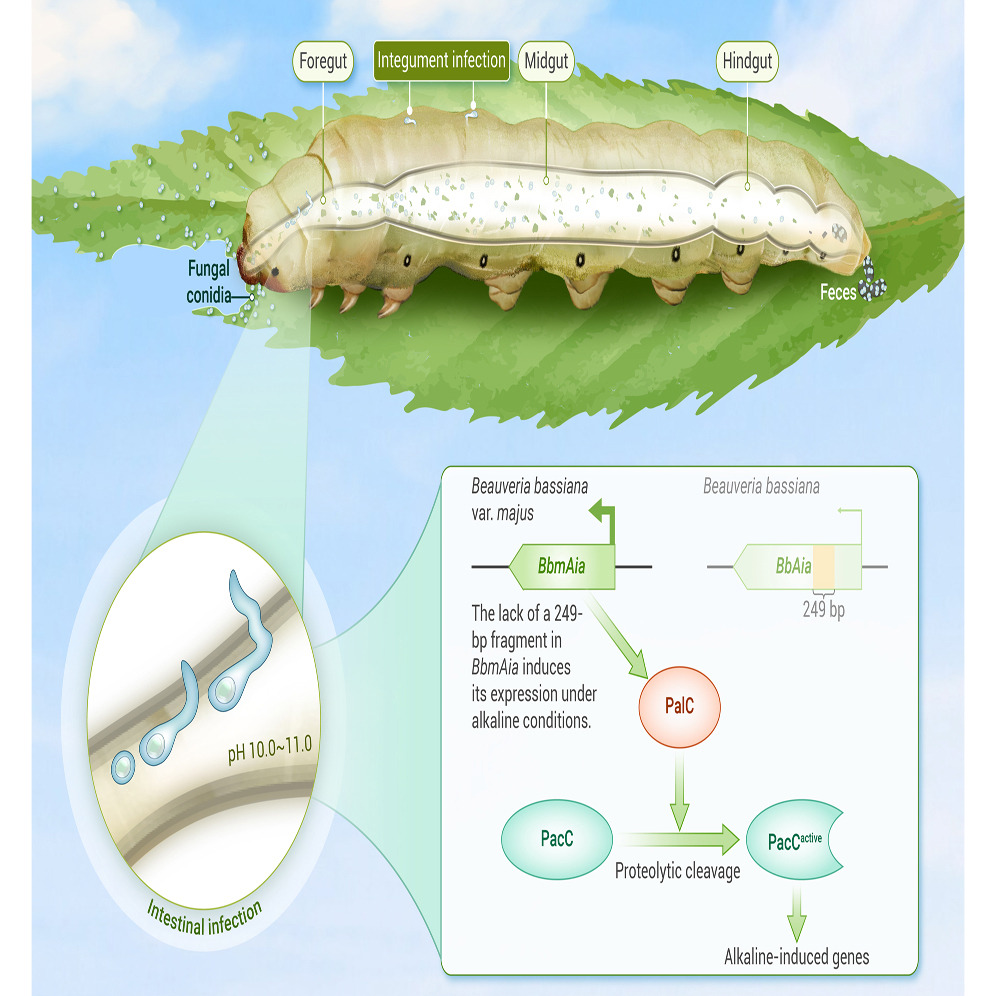
We report a new variety, Beauveria bassiana var. majus (Bbm), that can infect insects through the previously unrecognized foregut. The pH-responsive transcription factor PacC in Bbm exhibits rapid upregulation and efficient proteolytic processing via PalC for alkaline adaptation in the foregut. Expression of PalC is regulated by the adjacent downstream gene Aia. Compared to non-enteropathogenic strains such as ARSEF252, Aia in Bbm lacks a 249-bp fragment, resulting in its enhanced alkaline-induced expression. This induction promotes PalC upregulation and facilitates PacC activation. Expressing the active form of BbmPacC in ARSEF252 enables intestinal infection. This study uncovers the pH-responsive Aia-PalC-PacC cascade enhancing fungal alkaline tolerance for intestinal infection, laying the foundation for developing a new generation of fungal insecticides to control destructive insect pests.
Lai, Y., et al. (2024). Unveiling a novel entry gate: Insect foregut as an alternative infection route for fungal entomopathogens. The Innovation, 5(4). https://doi.org/10.1016/j.xinn.2024.100644
Pathogen-mediated RNAi and host immunity resposne
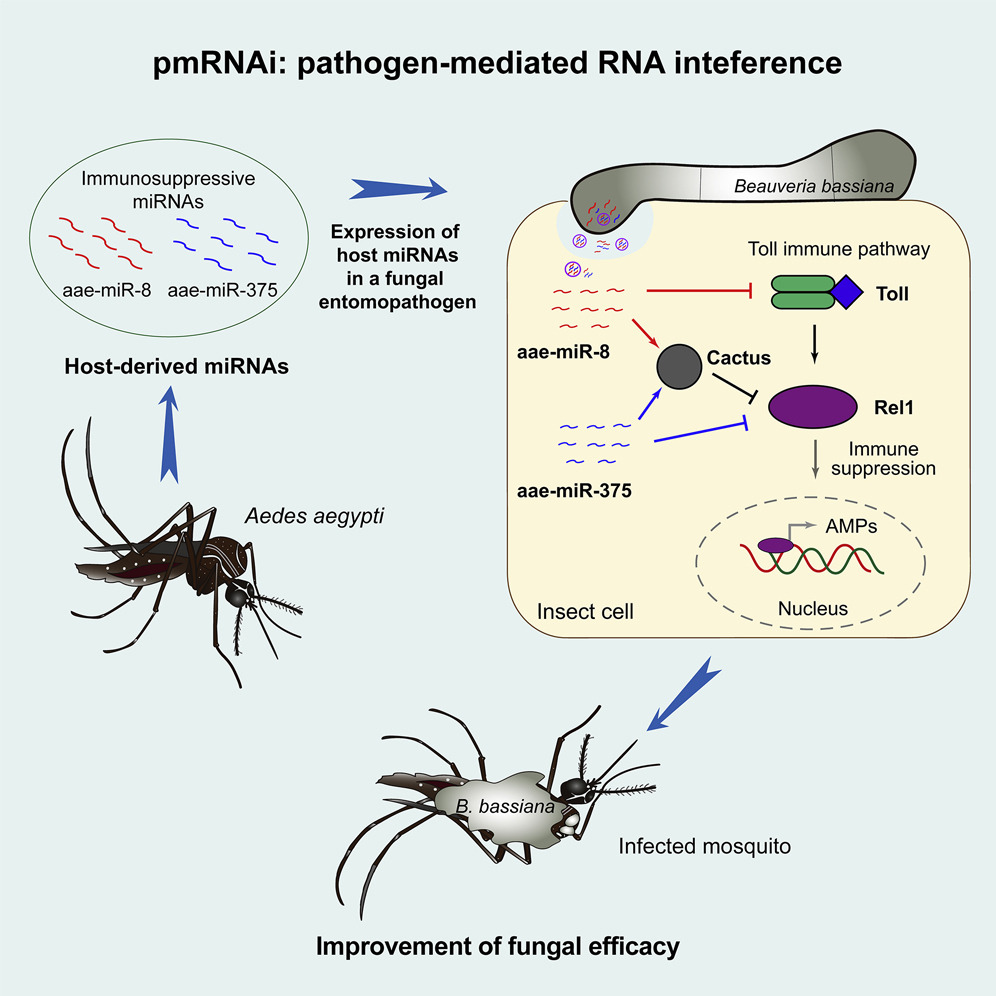
We engineered Beauveria bassiana to express Aedes immunosuppressive microRNAs (miRNAs) to induce host RNA interference (RNAi) immune responses. We show that engineered strains can produce and deliver the miRNAs into host cells to activate cross-kingdom RNAi during infection and suppress mosquito immunity by targeting multiple host genes, thereby dramatically increasing fungal virulence against Aedes aegypti and Galleria mellonella larvae. Importantly, expressing host miRNAs also significantly increases fungal virulence against insecticide-resistant mosquitoes, creating potential for insecticide-resistance management. This pathogen-mediated RNAi (pmRNAi)-based approach provides an innovative strategy to enhance the efficacy of fungal insecticides and eliminate the likelihood of resistance development.
Cui, C., et al. (2022). Expression of mosquito miRNAs in entomopathogenic fungus induces pathogen-mediated host RNA interference and increases fungal efficacy. Cell Reports, 41(4).